PRIN2022 - Reprogramming the ovarian tumor microenvironment by interfering with epigenetic self-renewal circuits centered on OCT4 and trans-acting OCT4 pseudogene lncRNA (OCT4TME)

Titolo progetto: PRIN2022 - Reprogramming the ovarian tumor microenvironment by interfering with epigenetic self-renewal circuits centered on OCT4 and trans-acting OCT4 pseudogene lncRNA (OCT4TME)
Programma di finanziamento: PRIN 2022
Responsabile scientifico: prof.ssa Roberta Benetti
Ruolo del DAME: partner
Descrizione generale:
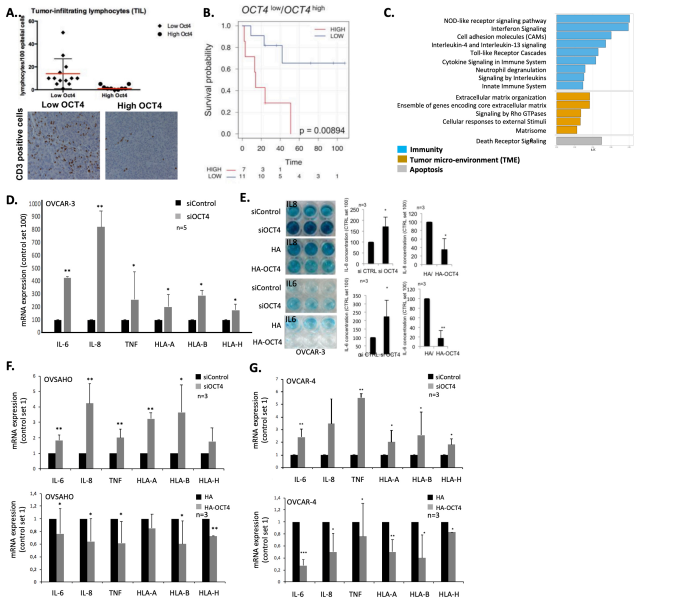
Epithelial ovarian cancer (EOC) is the most lethal gynecological cancer with > 60.000 new cases per year in the US and EU and single therapy that is beneficial for all EOC patients is currently not available. We found that selfrenewal circuits based on the self-renewal transcription factor OCT4 enhance ovarian cancer aggressiveness by programming a tumor promoting microenvironment. In this context OCT4 is tightly regulated by a derivative pseudogene lncRNA that deposits epigenetic silencing complexes to the OCT4 promoter using an unprecedented epigenetic mechanism, impinging on ovarian cancer aggressiveness. This highlights the relevance of tight regulation OCT4 dependent cancer pathways to program a tumor microenvironment (TME) in ovarian cancer. With this proposal we want to understand how OCT4 dependent gene expression signatures program a tumor-promoting TME and unravel the detailed mechanism by which the human OCT4 pseudogene 3 lncRNA (hOCT4P3) targets silencing complexes to the OCT4 promotor and identify alternative target regions across that ovarian cancer genome. In particular, we want to reach the following goals: 1. Build a EOC specimen collection stratified for hOCT4P3 and OCT4 and define cellular TME components by IHC. 2. Define OCT4 dependent gene expression signatures and identify compounds that revert OCT4 driven pathways of TME programming and EOC aggressiveness 3. Unravel central mechanisms of OCT4 driven EOC cell – TME communication 4. Define sequence-dependent and biophysical mechanism underlying hOCT4P3 lncRNA directed deposition of silencing complexes 5. Map hOCT4P3 lncRNA target sites in the EOC genome and integrate with gene expression data to understand a role of hOCT4P3 in modulating EOC aggressiveness The collaborative effort will provide new insights into the evolution of novel, clinically relevant epigenetic mechanisms and provide new inroads in disrupting a tumor promoting TME in ovarian cancer.
Partner del progetto:
- Università degli Studi di Udine
- Università degli studi di Trieste
Date inizio e fine progetto: 18.10.2023 – 17.10.2025
Budget totale del progetto: 106.761,00€
Sito web: https://prin.mur.gov.it/
Finanziato dall’Unione europea – Next Generation EU